How can we distinguish the following compounds from each other?
#A)# #"CCl"_4# and #"C"_4"H"_10#
#B)# 1-propanol and 2-methylpropan-2-ol
#C)# pentanamine and pentane
#D)# pent-1-ene and benzene
1 Answer
A)
Tetrachloromethane or Carbon tetra chloride (
So testing only the inflammability they can easily be distinguished
B) 1-propanonl(
2-methylpropan-2-ol (
Lucas' reagent is a solution of anhydrous zinc chloride in concentrated hydrochloric acid. This is used to distinguish between primary,secondary and tertiary alcohol of lower molar masses.
They all produce alkyl halides when treated with this reagent through
In case of 2-methylpropan-2-ol the turbidity appears almost immediately but in case of 1-propanonl turbidity appears after long time.
C) Pentanamine
It is soluble in dilute
and pentane
It is insoluble in dilute
In carbileamine test the comound under test is treated with chloform and alcoholic
Reaction
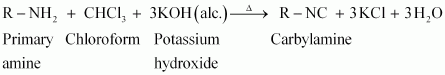
D). Pent-1-ene
and benzene
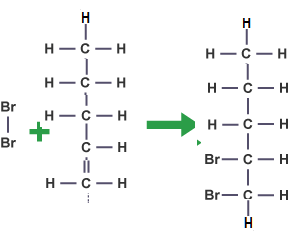
Both the compounds are unsaturated but the second one being aromatic one it is more stable and does not respond to test of un-saturation like dicolorization of red bromine water as done by the first one.
Reaction