Question #86320
1 Answer
Sep 20, 2017
You get 3-methylpentan-3-ol as the product.
Explanation:
Grignard reagents convert esters to tertiary alcohols.
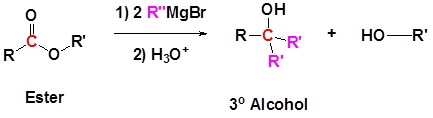
Thus, we predict the product to be 3-methylpentan-3-ol.
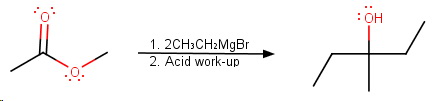
Here are the steps in the mechanism.
Step 1. Nucleophilic attack on the carbonyl group
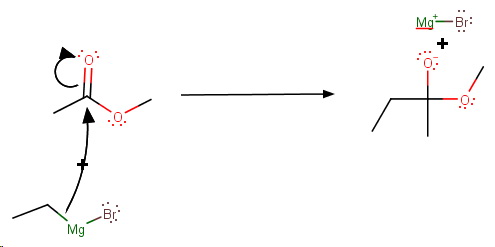
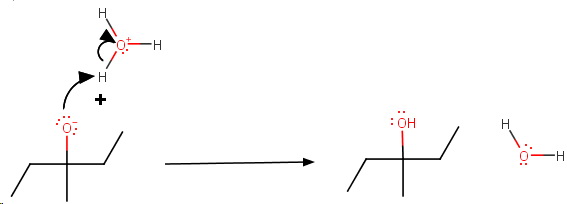
Step 2. Loss of the methoxide ion
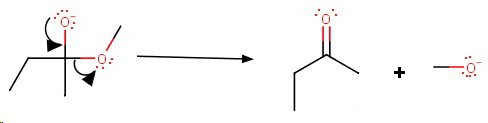
Step 3. A second nucleophilic attack by the Grignard Reagent
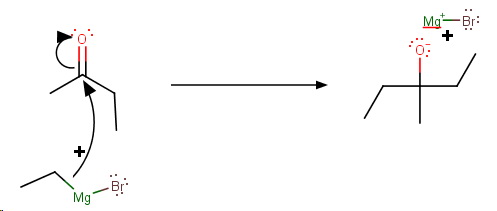
Step 4. Protonation of the alkoxide