Why are there many representations of benzene?
1 Answer
Well, you can draw resonance structures of many molecules.......
Explanation:
Of which the most obvious example is benzene.....
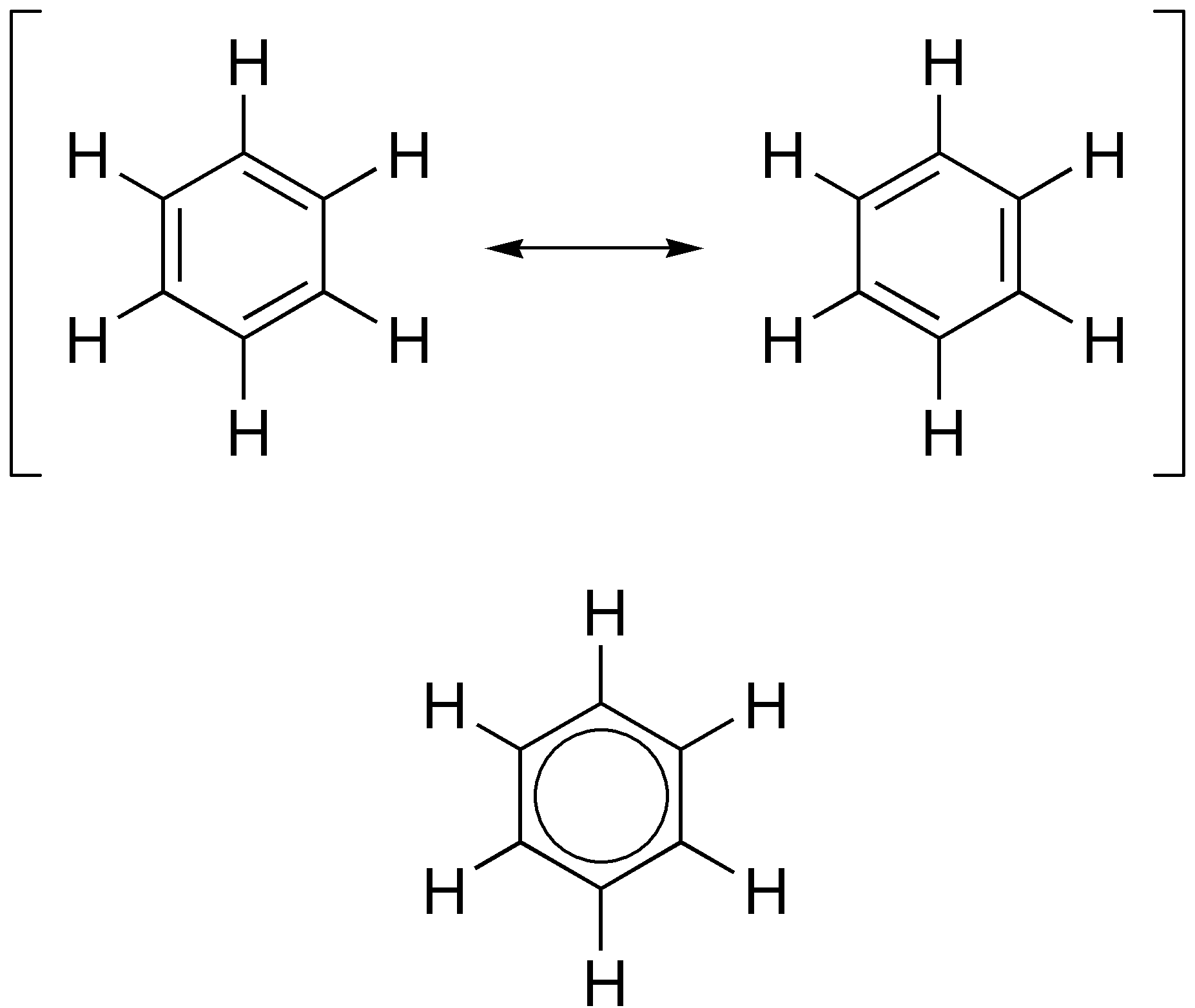
The second representation, the circle inscribed in a hexagon, is the more common representation these days (and these representations and arguments go thru fashions....) And when you see an organic professor drawing resonance structures on a blackboard or on a whiteboard you often get the idea that the prof can write 30 resonance structures per minute, whereas a student can only draw 5 resonance structures.
The use of resonance structures is as a simplification of observed patterns of reactivity; i.e. it is a formalism that has marginal physical significance, and yet it is useful for purposes of pedagogy. Once the benzene ring is substituted, the use of resonance isomers can help to rationalize (and to predict) observed experimental outcomes.

