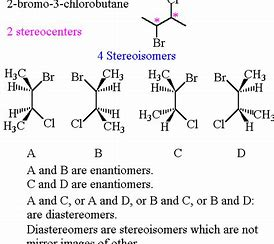
What is the relationship between #B# and #D#?
1 Answer
Are these not diastereomers.....?
Explanation:
As a tip, when you have represented a chiral structure CORRECTLY on the page, i.e. you know in the usual way... two substituents lie in the page, and one substituent projecting INTO the page, and one substituent projecting OUT of the page....the interchange of ANY two substituents results in the enantiomer....interchange again, and it need not be the original two substituents, and you get the enantiomer of an enantiomer, i.e. the mirror-image of a mirror-image, that is the ORIGINAL molecule. With me?
In your diagram you gots 2 chiral centres on each sub-diagram....had you interchanged 2 centres you would get the enantiomer of the original molecule. But here you interchanged ONE...i.e. at the benzyl position, and so we gots diastereomers.
Please don't despise the use of models to inform your reasoning (I acknowledge that I did not use a model, so it is

