What is pyramidal inversion?
1 Answer
Sep 8, 2017
It's a quantum tunnelling effect you would see in
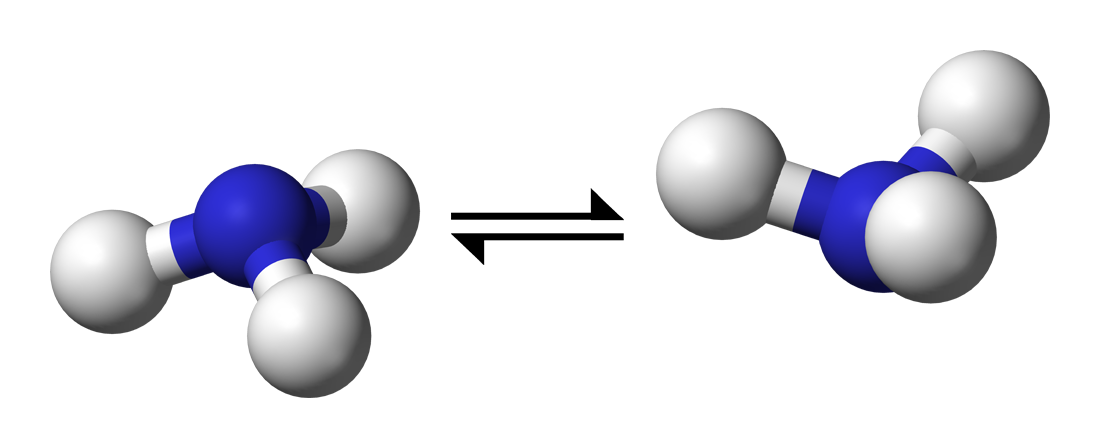
This tunnelling is rapid in
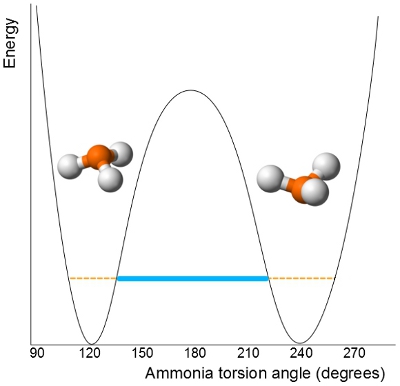
It passes through a planar transition state before it then inverts its trigonal pyramidal molecular geometry.

