Question #e2a2d
1 Answer
Band theory is the most useful, simplest piece of physics I ever learned.
Explanation:
Not sure how deep you want to go, so I’ll give a simple answer to start with, a couple of references and let you mull it over. If you have further questions, just ask - several contributors on here are expert in this field.
OK. Electrons in solids are bound to their parent atoms by varying degrees, but in our terms what matters is the energy levels they inhabit. The most tightly bound are said to be in a valence band and are fixed in most cases. At higher energies more loosely bound electrons partly fill a conduction band and these are free to move when propelled by an electric field.
Between the two bands lies an energy gap which prevents (in most cases) electrons transferring from one band to another. Finally, in this introductory part I should mention the Fermi level, which is the highest filled energy level at a particular temperature.
A diagram will help to visualise this:
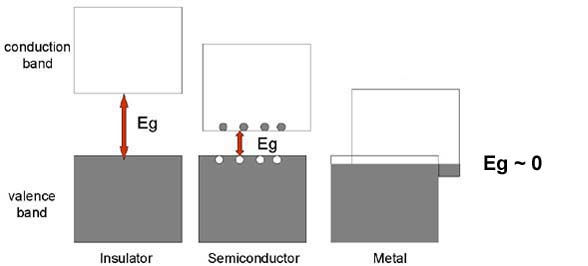
We define three basic classes of solids - insulators, in which the valence band is (generally) filled with electrons and the conduction band which is empty. The Fermi level lies above the top of the conduction band but well below the conduction band. The gap in these materials is wide enough that it prevents any electrons from transferring until exceptionally high temperatures, which means the valence electrons are ripped out, physically destroying the material.
Clearly, insulators do not conduct until a field is applied that is high enough to ruin the material, generally with sparks & fire risk.
Secondly, there are conductors where the Fermi level lies close to the bottom of the conduction band, meaning this band is partially filled at ‘thermal’ temperatures (room temp. approx.) In this case, even a tiny field will set electrons moving, causing a current to flow.
Most interesting are the semi-conductors. Semi-conductors have a narrow band gap with the Fermi level positioned between the two energy bands. This means that only a very few electrons exist in the upper band at room temperatures, but they have a good chance at moderate temperatures to cross the band gap.
Passing a current through materials like these tends to cause collisions with the lattice atoms, causing them to vibrate i.e. the material heats up. This causes promotion of large numbers of electrons, which means they conduct much more easily. So their resistance falls dramatically with increasing current, or temperature.
These materials are subdivided into intrinsically and extrinsically doped classes which allows electrons to “jump” the gap more easily and makes them much more useful in practice. I’ll leave this for now ... it really is a whole new topic!
The best reference to start with is probably this: http://hyperphysics.phy-astr.gsu.edu/hbase/Solids/band.html
A Fullerton explanation is given in the Wikipedia article here: https://en.m.wikipedia.org/wiki/Band_diagram

