Will #"2-pentylene"# generate geometric isomers?
1 Answer
Oct 16, 2017
Certainly,
Explanation:
And thus....the trans isomer....
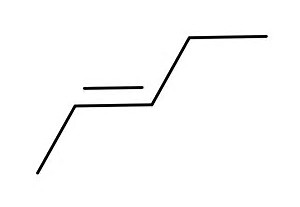
And the cis isomer....
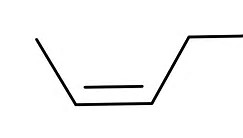
And these are GEOMETRIC isomers with distinct physical properties even given their identical
And for
And
How do you know? Write the molecule out as a line drawing, and use molecular models if you are unsure.

