Given the illustration, what is the activation energy for the transition #BrarrA#?
2 Answers
Oct 23, 2017
Well, what is
Explanation:
We assess the transition
You answered the wrong question.
Explanation:
You gave the activation energy for the reaction going from A to B.
The question asked for the activation energy for the reaction going from B to A (the reverse direction).
Ea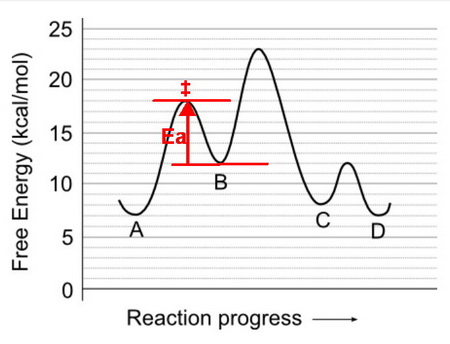
Going from B to A, you are starting at 12 kcal/mol and moving to the transition
state


