What are the symmetry elements and point group of #"CF"_4#, #"CClF"_3#, and #"CBrClF"_2#? What symmetry elements are lost in the descent from #"CF"_4# to #"CClF"_3# and then to #"CBrClF"_2#?
1 Answer
Warning! Long Answer.
1. 4
2. 1
Explanation:
Point group for
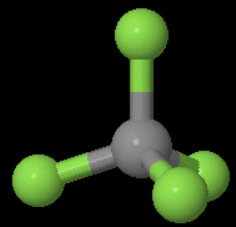
It has four
(a)
If you place the model on a table and look "down" the vertical
You can rotate the molecule 120°# about the axis and get an indistinguishable configuration of the molecule.
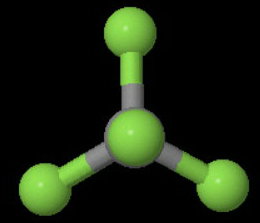
(b)
The image below shows one of the four
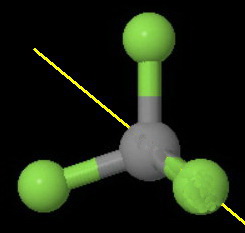
The axis bisects a
You can rotate the molecule 180° about this axis and get an indistinguishable configuration of the molecule.
(c)
The image below shows the two of the six
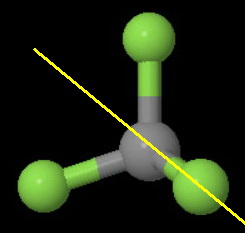
One is the plane of the screen. The other is a plane perpendicular to the screen and bisecting an
(d)
An
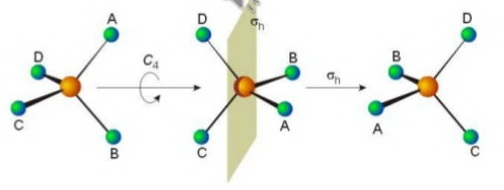
(Adapted from slideshare.net)
There are four of these axes in the molecule.
The structure of
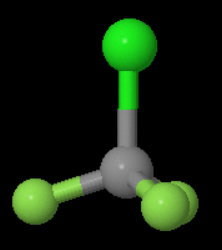
We see that the molecule has a
The three-fold axis is more obvious if you look down the
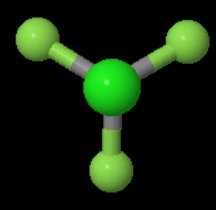
You can rotate the molecule 120° left or right and get a new configuration that is indistinguishable from the original.
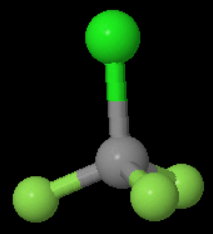
Each
A reflection converts the molecule into an indistinguishable form of the original.
In going from
**
The structure of the molecule is
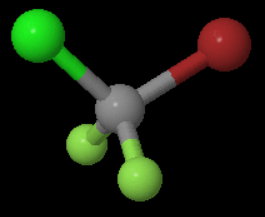
Thus, it is in point group
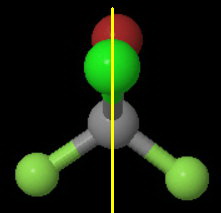
The plane contains the
One half of the molecule is a mirror image of the other half.
Thus, in going from

