Question #4c48b
1 Answer
Dec 10, 2017
Here's what I get.
Explanation:
The Dieckmann cyclization is a base-catalyzed intramolecular condensation of a diester to give a cyclic β-keto ester.
It is the intramolecular version of a Claisen condensation
Five- and six membered rings are are sterically favoured, so 1,6-diesters form five-membered rings, as in the animation below.
The reaction with 1,7-diesters gives six-membered rings.
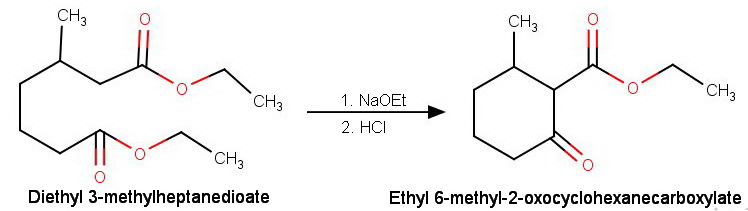
If the diesters are unsymmetrical, we get two different β-keto esters.
For example, the Dieckmann condensation of diethyl 3-methylheptadienoate can occur in two ways.
(a) The enolate ion formed at
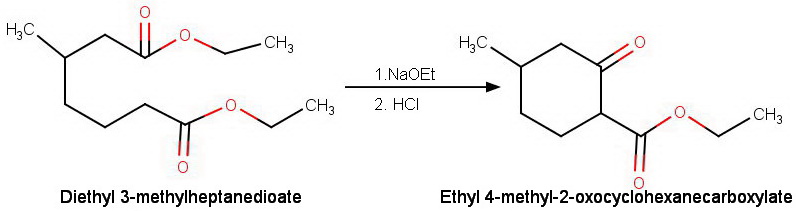
(b) The enolate ion formed at