We gots the interhalogen #ClF_3#; and clearly there are #4xx7=28*"electrons"#, i.e. 14 electron pairs to distribute over 4 centres....
Each fluorine atom possesses 3 non-bonding pairs, and this leaves 5 pairs of electrons to distribute around the central chlorine atom; 2 of these are non-bonding pairs, and there are three #Cl-F# bodning pairs. These electron pairs arrange themselves in a trigonal bipyramid....#/_F-Cl-F=180^@,90^@# to a first approximation.
But we describe molecular geometry on the basis of atomic, NOT electronic geometry. The molecule is thus #"T-shaped"#....
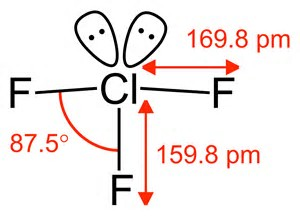
And with these #AX_3E_2# type molecules the lone pairs typically occupy the equatorial sites. Because this is a conformationally mobile, trigonal bipyramidal geometry, axial and equatorial fluorines would interchange at room temperature....and we would have to chill the molecule to freeze out the #"T-shaped"# stucture...and conceivably we could use #""^19F# #"NMR spectroscopy"# to do so...


