How do you draw the Lewis structure of carbonate dianion?
1 Answer
Dec 14, 2017
You mean of carbonate anion....
Explanation:
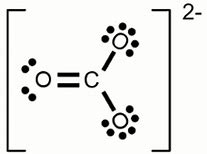
You mean of carbonate anion, i.e.
Carbonate anion is composed of 4 atoms, and we conceive the negative charge to lie on the most electronegative species, i.e. on the oxygen atoms....
A Lewis structure of

