What is the product for the hydrochlorination of 1-nitrocyclohexene?
1 Answer
Dec 20, 2017
I predict that the product will be 1-chloro-2-nitrocyclohexane.
Explanation:
We would expect
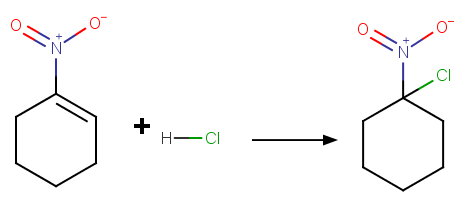
However, this mechanism puts a positive charge on the carbon atom next to another positive atom:
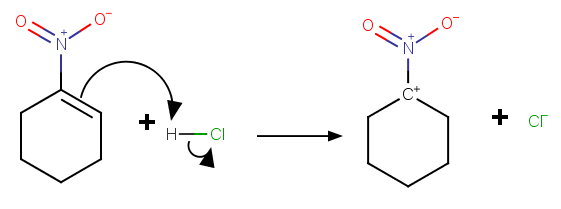
In this case, the addition is more likely to be anti-Markovnikov.
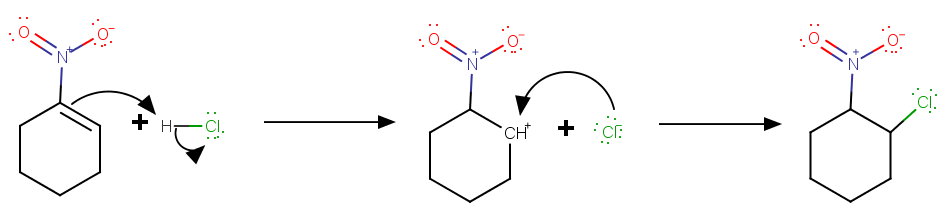
This avoids two adjacent + charges in the intermediate and forms a secondary carbocation.
I predict the product will be 1-chloro-2-nitrocyclohexane.

