Question #51785
1 Answer
Here's one way to do it.
Explanation:
Step 1. Draw a cyclohexane chair
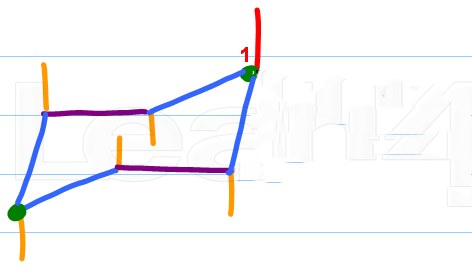
(Adapted from Leah4sci)
I like to start by drawing the axial bonds and numbering the top carbon as #"C1".
Step 2. Attach the isopropyl group
The bulkiest group always goes in an equatorial position,
Isopropyl is bulkier than ethyl, so we put the isopropyl group equatorial ("up") on
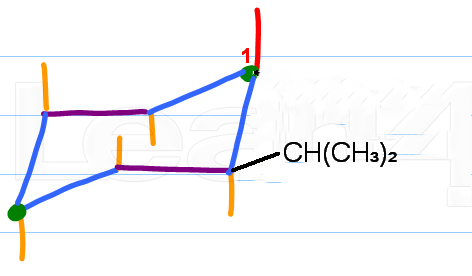
(Adapted from Leah4sci)
Step 3. Attach the ethyl group
The ethyl group is trans to isopropyl.
Isopropyl is "up", so ethyl must be down.
Put the isopropyl group in the equatorial position on
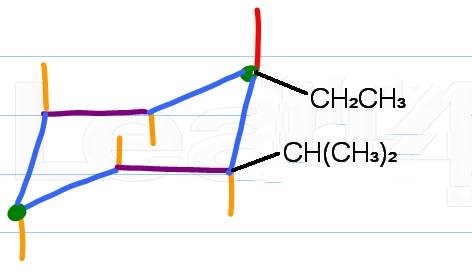
(Adapted from Leah4sci)
Step 4. Clean up the structure
Erase the axial bonds on the unsaturated
Also, put
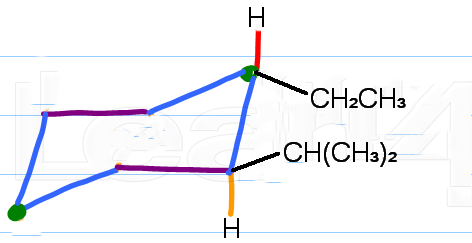
(Adapted from Leah4sci)

