Question #a3dc1
2 Answers
The numbers will be based on the location of unsaturation (e.g. the double bond) in the molecule. You never specified a location. Generally when accounting for "numbers" we count so the (i) functional group, (ii) double, and (iii) triple bonds have the lowest numbers in descending order, respectively. For instance,
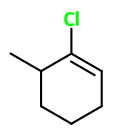
This may be 3-chloro-2-methylcyclohexene,

This may be 1-chloro-6-methylcyclohexene,

Big difference! Even if we flipped the molecule around, the two substituents are in opposite places.
Note: this is all hypothetical and I'm demonstrating why you may be incorrect. These could be wrong because you never supplied a bond-line drawing.
The correct name is 1-chloro-6-methylcyclohexene.
Explanation:
Are you asking about the correct numbering in this compound?
The wrong numbering
You could number the atoms this way:
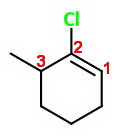
This gives the lowest sum of numbers, but that is INCORRECT.
The correct numbering
The double-bonded carbons must be numbered
If one of the
Then you number around the ring through carbon
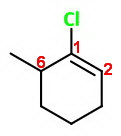
The correct name is 1-chloro-6-methylcyclohexene.


