Question #99dda
1 Answer
Jan 2, 2018
B. The carbonyl group of propanone is most polar.
Explanation:
The polarity of a carbonyl group is enhanced by hyperconjugation of the carbonyl π system with the
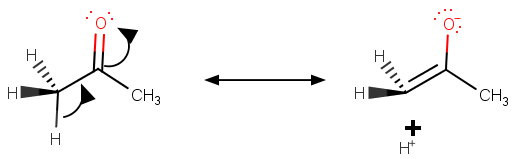
Propanone has six adjacent
Pentan-3-one has four adjacent
Ethanal has three adjacent
Methanal has no adjacent
This mirrors the observed order of dipole moments.

