Question #a92fc
1 Answer
Here's my explanation.
Explanation:
The nucleophile is not a bromine atom,
The nucleophile is a bromide ion,
An example is the use of hydrobromic acid to convert an alcohol to an alkyl bromide.
The final step in the mechanism involves the nucleophilic attack of a bromide on an alkyloxonium ion.
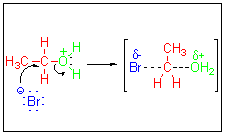
The electrophile is not a bromine atom. It is a bromine molecule,
An example is the bromination of an alkene.

The

