How much heat does a freezer remove when it converts 600 g of water at 21 °C to ice at -21 °C?
The specific heat capacity of water is # "4.184 J·°C"^"-1""g"^"-1"# .
The specific heat capacity of ice is #2.010 color(white)(l)"J"·"°C"^"-1""g"^"-1"# .
#Δ_text(freeze)H ="-334 J·g"^"-1"#
The specific heat capacity of water is
The specific heat capacity of ice is
1 Answer
The freezer must remove 271 kJ.
Explanation:
A schematic cooling curve for water is shown below.
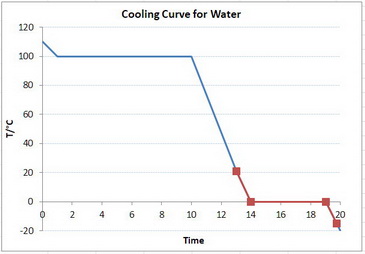
You are starting with the water at the top of the red graph, cooling it to its freezing point, freezing the water along the horizontal line, and then cooling the ice to the bottom of the red graph.
Thus, there are three separate heat removals involved in this problem:
#q_1# = heat removed by cooling the water from 21 °C to 0°C#q_2# = heat removed by freezing the water at 0 °C#q_3# = heat removed by cooling the ice from 0 °C to 258.15 K (-15.00 °C)
where
The freezer must remove 271 kJ.

