A certain element exists in a face-centered cubic structure. The atomic radius of an atom of this element is 185 pm. What is the density of this element in g/cm3?(Assume the element has a molar mass of 70.9 g/mol.)
A certain element exists in a face-centered cubic structure. The atomic radius of an atom of this element is 185 pm. Calculate the density of this element in g/cm3. (Assume the element has a molar mass of 70.9 g/mol.)
A certain element exists in a face-centered cubic structure. The atomic radius of an atom of this element is 185 pm. Calculate the density of this element in g/cm3. (Assume the element has a molar mass of 70.9 g/mol.)
1 Answer
The density of the element is
Explanation:
Calculate the mass of a unit cell

An fcc unit cell contains
Calculate the volume of a unit cell
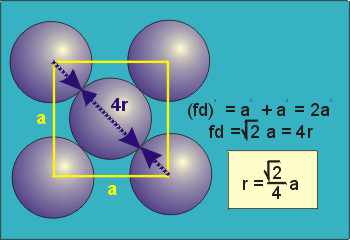
The diagonal along a face is
Calculate the density


