A chemist needs 140 milliliters of a 73% solution and has only 37% and 79% solutions available. How many milliliters of each should be mixed to get the desired solution?
2 Answers
I am going to show you two approaches
Method 1 of 2
120 ml of 79% concentration
Explanation:
There is a direct link in quantities of each the solutions.
If you have
So if we only consider the strongest solution we are directly inferring the weaker one.
If we have all the stronger solution the concentration is 79%
If we have all the weaker solution (none of the stronger) the solution concentration is 37%
Every other possible blend will be between. So we have the situation of:
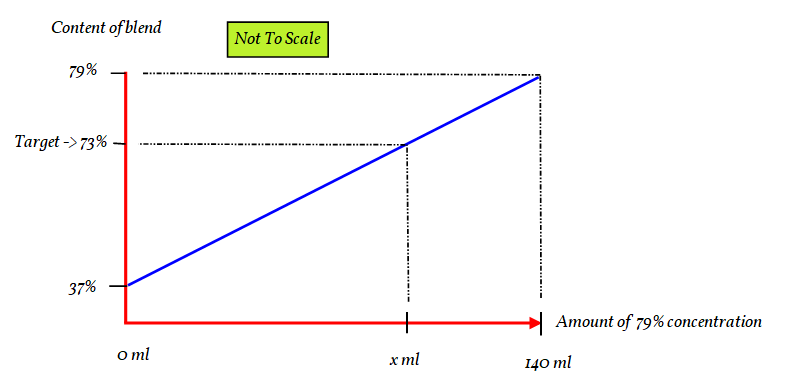
The slope of part is the same as the slope of all.
Turn it all upside down:
120 ml of 79% concentration
Method 2 of 2: have a look at method 1 first.
Explanation:
Let the amount of 79% concentration be
Let the amount of 37% concentration be
Multiply both sides by 100

