Are water's polarity and ability to form hydrogen bonds the same thing? Or are they only connected? Please explain in detail, thank you in advance!
1 Answer
They are only connected, given that there exist polar molecules that DO NOT exhibit hydrogen-bonding....
Explanation:
Hydrogen-bonding is a potent force of
For
And in the bulk phase, the individual dipoles can interact...
And such intermolecular interaction ELEVATES the boiling point, and we compare this to the hydrides of the lower elements in each Group.
Given that we are chemists, physical scientists, we should interrogate some data...
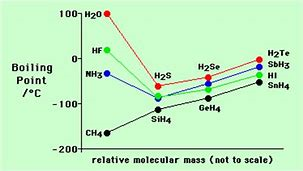
Are these data consistent with what we have argued? Why or why not?

