Balance the following reaction: #"__Fe +___NaBr"##rarr##"____FeBr"_3 + "___Na"#?
and what reaction would this be classified as?
-Synthesis
-Single Replacement
-Decomposition
-Combustion
-Double replacement
and what reaction would this be classified as?
-Synthesis
-Single Replacement
-Decomposition
-Combustion
-Double replacement
1 Answer
Single replacement (displacement) reaction format.
Explanation:
Balanced equation
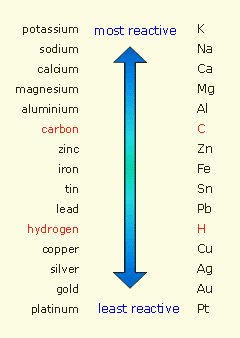
This is in the form of a single replacement (displacement) reaction. However, the elemental metal replacing the metal in the compound must be more reactive than the metal in the compound.
If you consult a metal reactivity series, you will see that iron (Fe) cannot replace sodium (Na) because it is less reactive than sodium. So this reaction would not take place.
The opposite reaction would occur.
A metal can only replace (displace) a metal below it in the series. Note that Na is above Fe in the series.