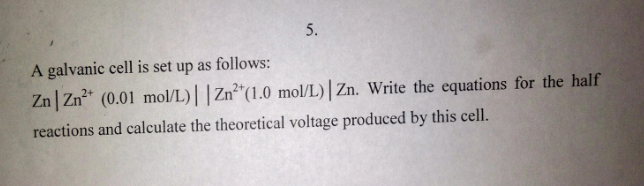Step 1. Write the half-reactions for the cell
We have here a concentration cell, In which the two half-cell reactions are the same, but the concentrations are different.
Because the two half-reactions are identical,
#E_text(cell)^@ = E_text(red)^@ - E_text(ox)^@ = 0#
Step 2. Write the cell reaction
The left-hand cell is the anode.
#"Anode:" color(white)(mml)"Zn" → "Zn"^"2+""(0.01 mol/L)"
+ "2e"^"-"#
#"Cathode":color(white)(m)ul("Zn"^"2+""(1.0 mol/L)" + "2e"^"-" → "Zn"color(white)(mmmmmmmmmm")#
#"Overall": color(white)(ml)"Zn + Zn"^"2+""(1.0 mol/L)"→ "Zn"^"2+""(0.01 mol/L)" + "Zn"#
Step 3. Calculate the cell potential
The cell is not at standard conditions, so we must use the Nernst Equation:
#color(blue)(bar(ul(|color(white)(a/a)E = E^° - (RT)/(zF)lnQcolor(white)(a/a)|)))" "#
where
#E^°# is the standard cell potential
#R# is the Universal Gas Constant
#T# is the temperature
#z# is the moles of electrons transferred per mole of copper
#F# is the Faraday constant
#Q# is the reaction quotient
Assume that the temperature is 25 °C.
#E^@ = 0#
#Q = (["Zn"^"2+"]_text(prod))/(["Zn"^"2+"]_text(react)) = (0.01 color(red)(cancel(color(black)("mol/L"))))/(1 color(red)(cancel(color(black)("mol/L")))) = 0.01#
#E = E^@ -(RT)/(zF)lnQ = -("8.314 V"·color(red)(cancel(color(black)("C·K"^"-1""mol"^"-1"))) × 298.15 color(red)(cancel(color(black)("K"))))/("2 × 96 485" color(red)(cancel(color(black)("C·mol"^"-1"))))ln(0.01) = "0.059 V"#