Can we treat stretch mode in vibrational analysis as Harmonic oscillator?
1 Answer
Sometimes, but it would usually be a crude approximation if too many atoms are bundled into one "effective atom" on either side of the oscillator, i.e. if you did benzene, then it wouldn't make much sense to treat one
For example, suppose we took methane and used the frequency of its
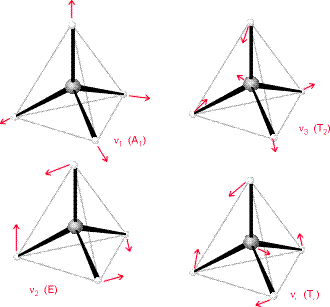
Let's pretend that the two hydrogens are one effective atom on each side, so that the reduced mass of each side is:
#mu = (m_1m_2)/(m_1 + m_2) = m_H^2/(2m_H) = m_H/2#
#= "0.0010079 kg/2"/"mol" xx ("1 mol")/(6.0221413 xx 10^23)#
#= 8.368 xx 10^(-28) "kg"#
And the collective reduced mass
#mu' = (mu^2)/(2mu) = mu/2 = 4.184 xx 10^(-28) "kg"#
We can then check if this is a reasonable approximation by using the actual vibrational frequency of
We don't expect this to give a good value.
#tildeomega_e = 1/(2pic) sqrt(k/(mu'))#
#=> k = 4pi^2c^2 tildeomega_e^2mu#
#= 4pi^2 cdot (2.998 xx 10^(10) "cm/s")^2("3104 cm"^(-1))^2(4.184 xx 10^(-28) "kg")#
#= "143.05 kg/s"^2#
The typical force constant of a bond in a diatomic molecule is a few hundred to a few thousand
However, this is unusually small, as it would suggest a weaker "bond" than
This is not usually done in practice, and this value has not been tabulated anywhere because it is not very useful.

