Catalysts are used to make a chemical reaction do what? A. Ignite B. Change C. Go faster D. Activate
1 Answer
Apr 21, 2018
Explanation:
A catalyst acts by altering the activation energy of a given reaction...and we can address a much-used graphic which plots energy versus reaction coordinate for catalyzed and non-catalyzed reactions.
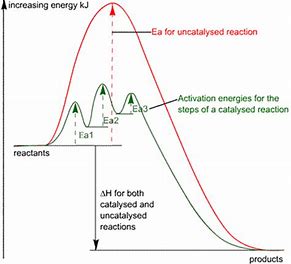
And thus both for FORWARD, and REVERSE reaction, the enthalpy of activation has been reduced. And so a greater proportion of reactant molecules have the necessary activation energy to undergo reaction. The catalyzed reaction is thus associated with a LOWER ACTIVATION energy, and thus a faster rate of reaction.

