Consider the molecule [MnX6]2+, where X is a neutral ligand. Assume that X is a strong field ligand?
1 Answer
DISCLAIMER: Long answer!
THE NUMBER OF 3D VALENCE ELECTRONS IN THE COMPLEX
When you examine the location of
Since
HIGH-SPIN VS LOW-SPIN, AS IT RELATES TO STRONG-FIELD AND WEAK-FIELD LIGANDS
A
We assume strong-field for
- A
#sigma# donor (such as#:"NH"_3# ,#"CO"# , or#""^(-)"CN"# ) - A
#pi# acceptor (such as#"CO"# or#""^(-)"CN"# ) - Both (such as
#"CO"# or#""^(-)"CN"# )
SIGMA DONORS
Let's say we just have a good
In this case, it destabilizes the
#e_(g)# orbitals (there are two of those orbitals), since those interact with the#sigma# bonding molecular orbitals brought in by the#sigma# donor(s).#"CO"# is an example:
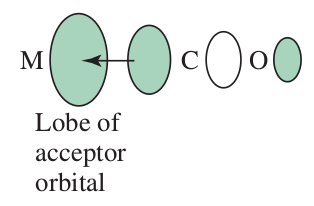
As a result, the two upper-energy (
#e_g# ) orbitals get higher in energy (relative to the free-ion energy), increasing the crystal-field splitting energy#Delta_o# (#Delta# in your diagram), promoting low spin.
PI ACCEPTORS
Or, let's say we have a good
In this case, the
#pi# antibonding molecular orbitals of the ligand accept electrons from the#3# lower-energy (#t_(2g)# )#3d# orbitals.
This stabilizes these
#3# lower-energy (#t_(2g)# )#3d# orbitals, decreasing their energy. This also increases#Delta_o# .
Since
CRYSTAL-FIELD SPLITTING DIAGRAM
When a transition metal complex has a coordination number of
The diagram you were given is the crystal-field splitting diagram for the five
Here is the filled diagram for a manganese(II) complex in high spin or low spin:
The difference is that for a low spin complex, which corresponds to having a strong-field ligand,
So the electrons can capably fill the
PI DONORS
A weak-field ligand contributes little repulsion. Actually, weak-field ligands are typically
If you have a
It has filled
#pi# bonding molecular orbitals that donate into the three (#t_(2g)# )#3d# orbitals and destabilize them, increasing their energy.That actually decreases
#Delta_o# , and promotes high spin.
So,
This diagram summarizes the trends for
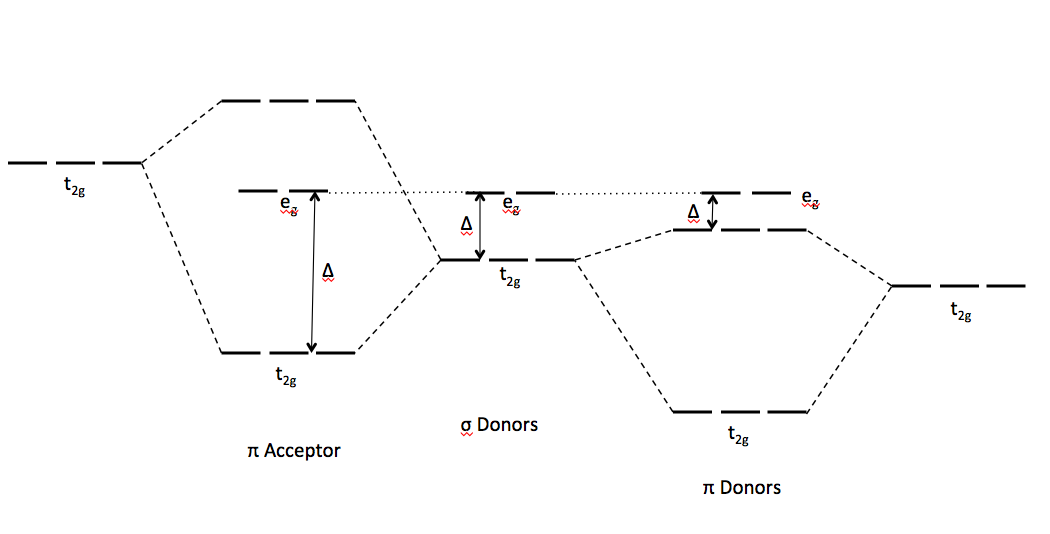
CRYSTAL-FIELD SPLITTING ENERGY
Since the light absorbed will promote electrons from the
Since
(Thus, you can also say that a longer wavelength is absorbed.)

