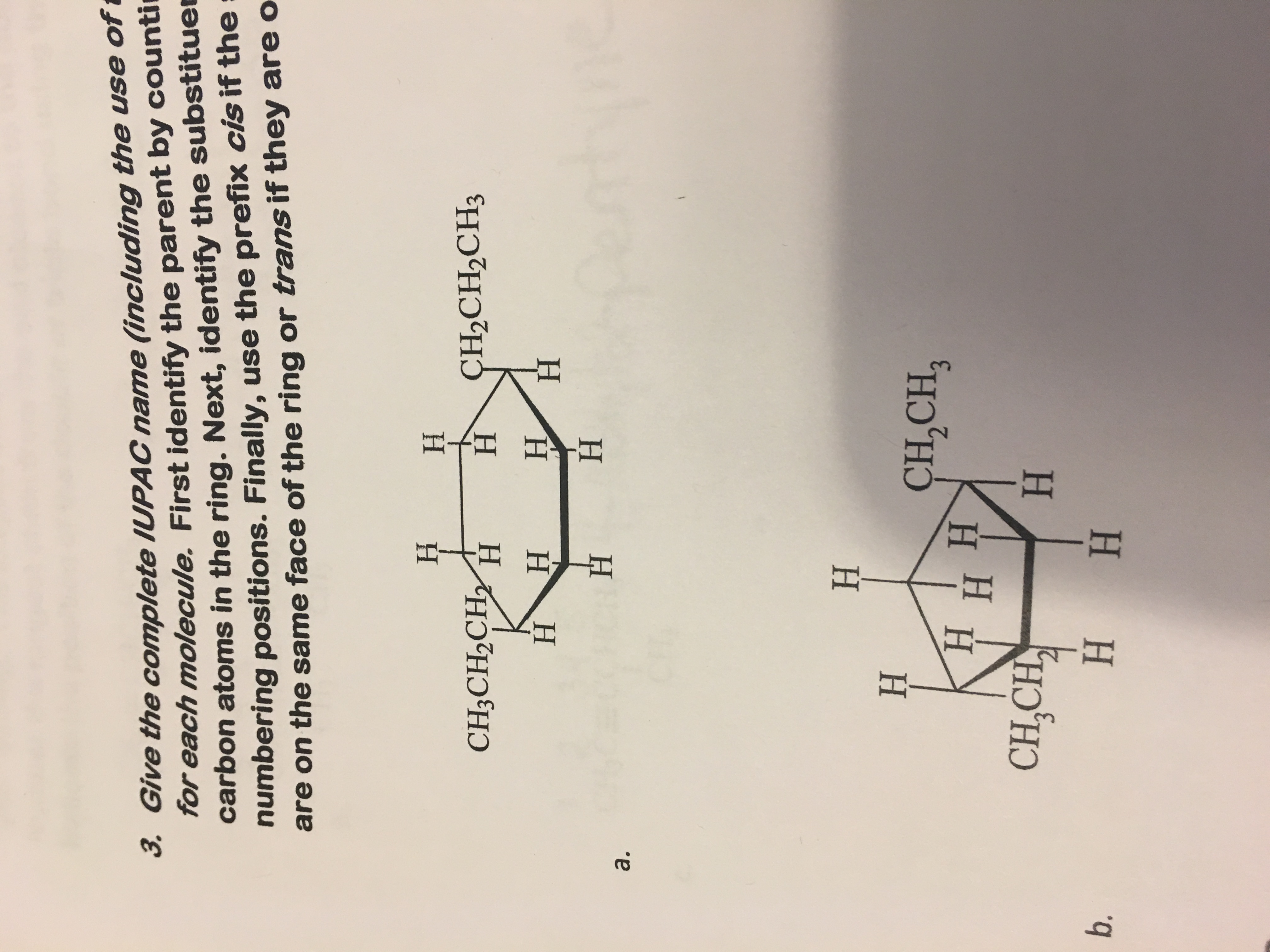
Could someone please tell me the IUPAC name for these?
I'M SORRY about the side ways picture, I've tried everything to get it straight.
I'M SORRY about the side ways picture, I've tried everything to get it straight.
1 Answer
(a)
I reproduced parts of your question below to make them more readable.
The compounds are both saturated cyclic hydrocarbons, so their class name begins with cyclo- and ends with -ane.
They are
(a) Name the first compound
(i) Count the number of carbon atoms in the ring.
There are six carbon atoms. The numerical prefix for "six" is hex-. The name becomes cyclohexane.
(ii) Number the carbon atoms in the ring
One substituent is automatically
Then number the other carbon atoms, taking the shortest path to the next substituent.
The substituents are on
(iii) Name the substituents
The substituents each have three carbon atoms.
The name of a carbon chain ends in -yl, and the numerical prefix for a three-carbon chain is prop-.
Each sidechain is a propyl group. The name becomes propylcyclohexane.
(iv) Indicate the numbers of each sidechain by a multiplying prefix.
There are two propyl groups, and the multiplying prefix for "two" is di-
The name becomes dipropylcyclohexane.
(v) Attach the numbers if the locants (the locating carbon numbers), separated from each other by commas and from letters by hyphens
The groups are on
(vi) Identify the cis-trans relationship of the groups
Both groups are "on top" of the ring — they are on the same side of the ring. The prefix for "same side" is cis-.
The complete name becomes
(b). Use the above principles to name the second compound
(i) Five carbon atoms in a ring → cyclopentane
(ii) Substituents on atoms 1 and 3.
(iii) Two-carbon chains → ethyl → ethylcyclopentane
(iv) Two ethyl groups → diethylcyclopentane
(v) Add locants → 1.3-diethylcyclopentane
(vi) Add stereochemistry
One group is "on top" of the ring, and the other is "on the bottom." The prefix for "on opposite sides" is trans-.
The complete name becomes

