Do electrons exist in fixed orbits, where they cannot move from one level to another?
1 Answer
Oct 30, 2015
No. Valence electrons can absorb energy and enter an excited state.
Explanation:
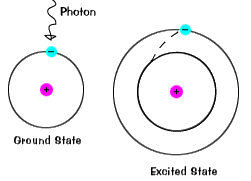
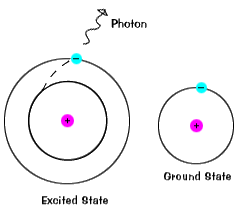
The valence electrons, which are the electrons in the outermost (highest energy) s and p orbitals, can absorb energy from heat or light. The excited electron jumps to an unoccupied higher energy orbital. This is called an excited state. This is an unstable condition and the excited electron drops back to its ground state, during which energy is emitted in the same quantity as was absorbed. This explains the results of the flame tests, how neon lights work, and how fluorescent lights work.
.

