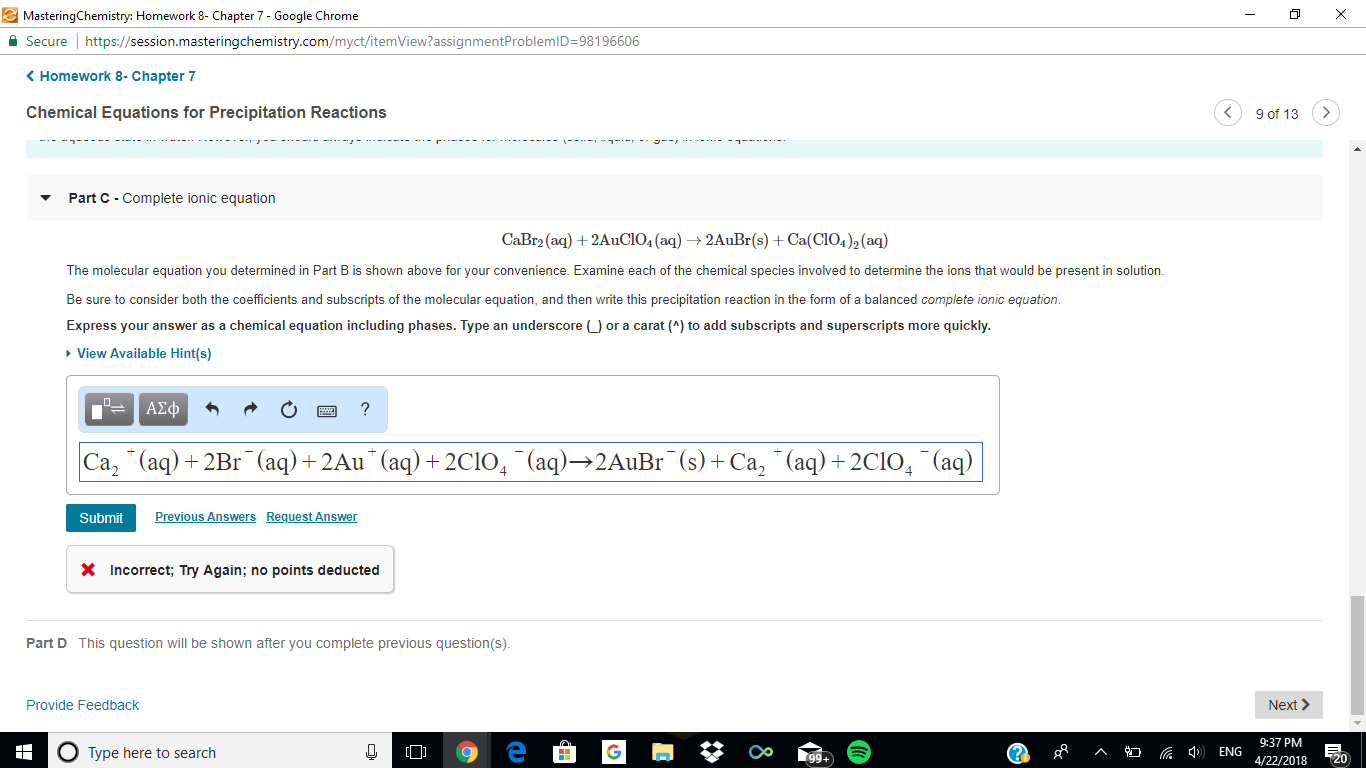
Examine each of the chemical species involved to determine the ions that would be present in solution?
1 Answer
Apr 28, 2018
Here's what I get.
Explanation:
Your molecular equation is
To generate the ionic equation, we write the formulas of the soluble ionic compounds as separate ions.
We do not write the formulas of solids as ions because the ions are locked in the solids and do not get into the solution.
We get
The gold(I) and bromide ions combine to form the precipitate, while the calcium and perchlorate ions remain in solution as spectator ions.

