Explain in detail how is furan is aromatic?
1 Answer
May 24, 2018
Well, a molecule is aromatic if it has
Explanation:
And we look at
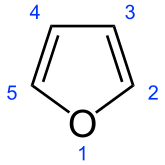
There are FOUR
Well, a molecule is aromatic if it has
And we look at

There are FOUR