Find density with given edge length?
There is a compound X composed of zinc and sulfur atoms. The edge length of the cell is 5.411Å. I need to calculate the density of compound X.
I try to use edge length to calculate the volume=(edge length)^(3)
However, i am stuck on the calculation of the mass.
How should I do this?
There is a compound X composed of zinc and sulfur atoms. The edge length of the cell is 5.411Å. I need to calculate the density of compound X.
I try to use edge length to calculate the volume=(edge length)^(3)
However, i am stuck on the calculation of the mass.
How should I do this?
1 Answer
We ASSUME that we have a cubic unit cell....
Explanation:
AND such a cubic cell contains FOUR formula units of
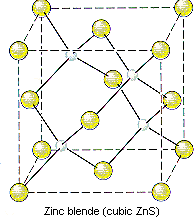
Eight zinc atoms at the corners, four zinc atoms at the faces, and one at the center gives
And thus FOUR formula units of zinc sulfide has a mass of ...
And all I have done is divide the molar mass by the Avocado number to get the mass of a SINGLE formula unit.
And so to take the density, we simply take the quotient...
The calculation is in the right order of magnitude. I would suspect


