Help physics ?
Nitrogen weighing 9 g is in a cylinder with vertical walls under the piston with weight. Piston area S = 90 cm². After heating
gas at ΔT = 25K, the piston rose to a height of H = 7 cm. Find the mass of the piston with the girk. The external atmospheric pressure p (a) = 10 ^ 5 Pa. Friction of the piston against the cylinder wall is neglected
Nitrogen weighing 9 g is in a cylinder with vertical walls under the piston with weight. Piston area S = 90 cm². After heating
gas at ΔT = 25K, the piston rose to a height of H = 7 cm. Find the mass of the piston with the girk. The external atmospheric pressure p (a) = 10 ^ 5 Pa. Friction of the piston against the cylinder wall is neglected
1 Answer
Explanation:
See the diagram,use it to understand,why the piston was at rest before heating the gas,well it was such because,the downward force exerted on the
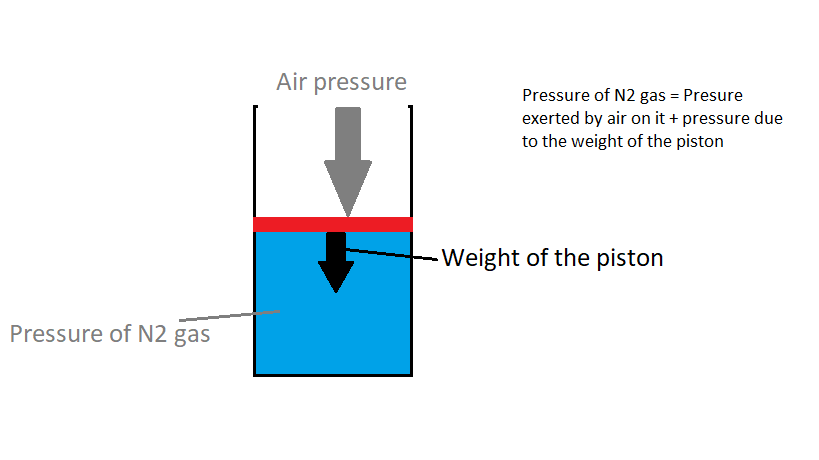
When,the gas was heated,some work was done by the gas,as a result of which it expanded(volume increased at constant pressure) and the piston was pushed upwards,
So,work done by the gas =
Again,work done by the gas in moving the piston upward by
=
So,
or,
Given,
So,
So,mass of the piston is

