How can steareoisomerism occur in alkenes?
1 Answer
Mar 6, 2017
Consider
Explanation:
Is this molecule.........
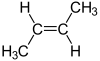
the same as this molecule.......?
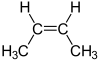
Do you agree that they are not the same? Why not? Because certainly for each isomer the connectivity is the same:
Cis and trans butylene have identical connectivity BUT DIFFERENT geometries, and thus these are properly described as geometric isomers. The two isomers have different chemical and physical properties, and these start with boiling point.

