How can you distinguish between following pairs of compounds with the help of infrared spectroscopy? Acid chlorideCH3COCL and carboxylic acid CH3COOH
1 Answer
May 10, 2018
Should acetyl choride have an
Explanation:
The answer is no...and
From the web....
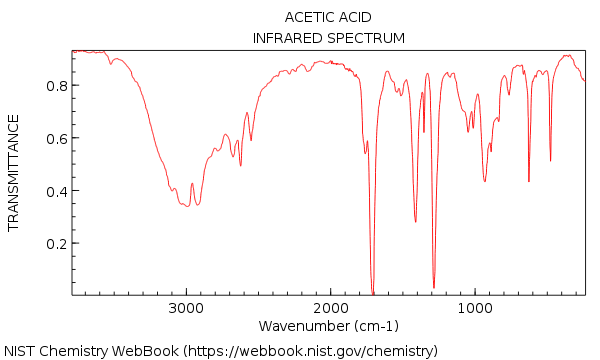
There is a whacking, great hydroxyl absorption at
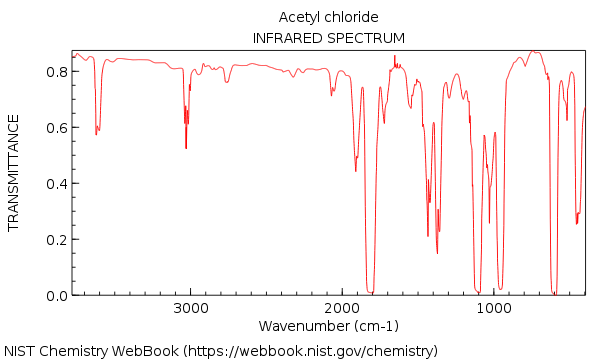
And thus acetyl chloride contains NO hydroxyl absorption, while retaining the carbonyl absorption at approx.

