How many isomers do molecules a) and b) have and can they be dissolved in acid enviroment?
a) molecule*HCl

a) molecule*HCl
1 Answer
DISCLAIMER: LONG ANSWER!
In
STEREOISOMERS OF (A) AND (B)
The number of stereoisomers we can have are
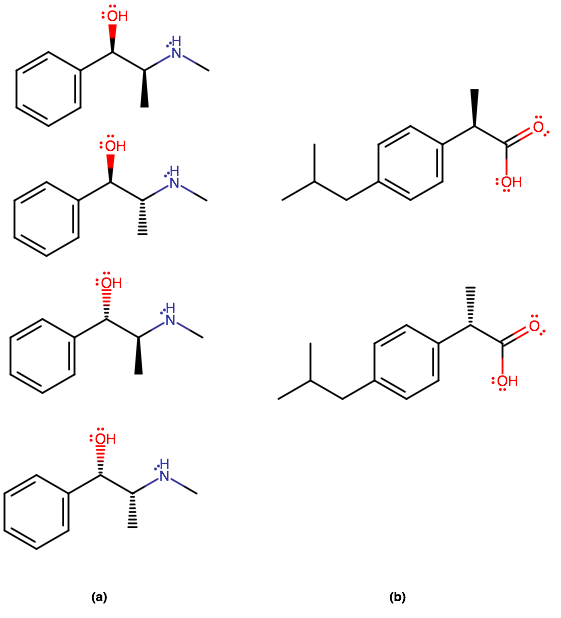
Let
#(a, i)# has two stereocenters. The left one has the following priorities:
#"OH" > "CHCH"_3"NH"- > "Ph" > "H"# ,#=> bb" R"# stereocenterwhere
#"Ph"# , phenyl, has a carbon center treated as having three "ghost carbons", as if it were#"C"-("CH"_3)_3# or#"C"-"C"-="C"-# .Atomic number takes preference in assigning priorities, followed by the highest quantity of substituents of the same atomic number.
And the right one has the following priorities:
#"NHCH"_3 > "CHOHPh" > "CH"_3 > "H"# #=> bb" S"# stereocenterThat makes
#color(blue)((a","i))# the#color(blue)(bb((R","S)))# stereoisomer.By comparison with
#(a,i)# , we therefore have that#color(blue)((a","ii))# ,#color(blue)((a","iii))# , and#color(blue)((a","iv))# are the#color(blue)bb((R","R))# ,#color(blue)bb((S","S))# , and#color(blue)bb((S","R))# stereoisomers, respectively.
#(b,i)# has one stereocenter, and its configuration from the above logic uses the following priorities:
#"COOH" > "Ph" > "CH"_3 > "H"# #=> bb(R)# stereocenterThus,
#color(blue)((b","i))# is the#color(blue)(bbR)# stereoisomer, and#color(blue)((b","ii))# is the#color(blue)(bbS)# stereoisomer.
SOLUBILITY IN ACID?
As for whether they can be dissolved in acid, it depends on what the
But the most convenient solvent with tabulated
A solution made more acidic helps maintain the present form of the species with
#"pH" = "pK"_a + log \frac(["A"^(-)])(["HA"])# ,
#"pH"# #<# #"pK"_a# implies#["A"^(-)] < ["HA"]# , where#"HA"# is either#(a)# or#(b)# in their present form.
So, you would need to know the
I searched these on Scifinder, and I would estimate the
However, if the solution is too acidic, the solvent itself may become protonated as well, making it a worse solvent for these compounds (which are not charged in their acidic form).

