How many resonating structures are there for the following compound? Also, draw their resonating structures?
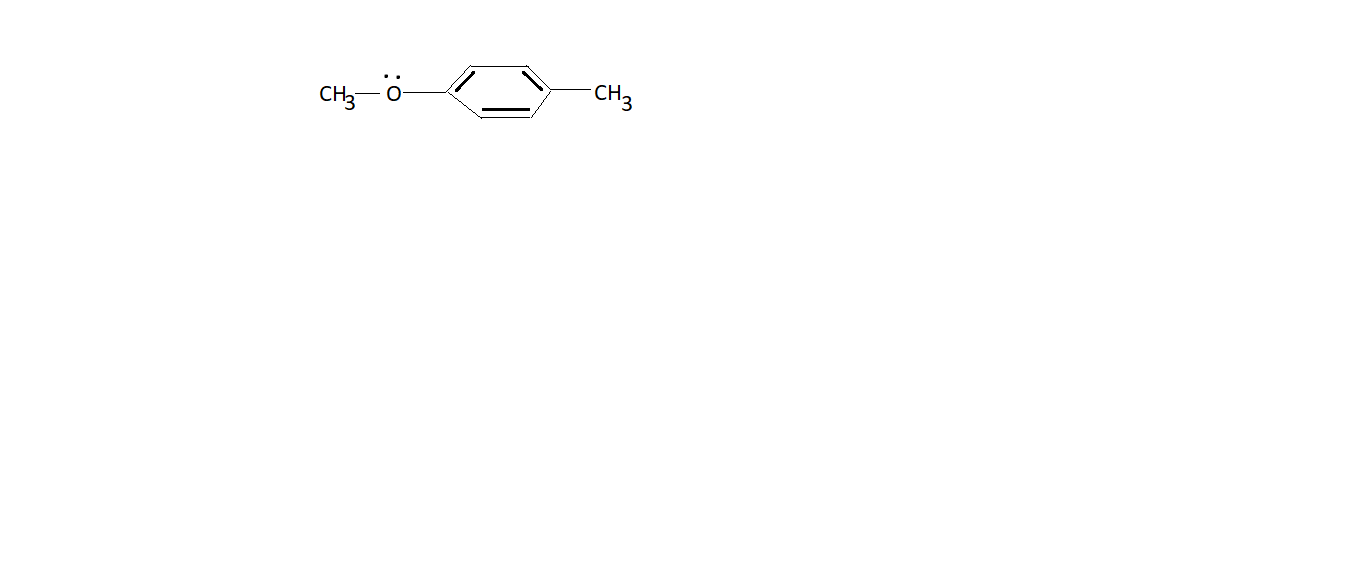
1 Answer
Mar 21, 2018
Well, look at the resonance structures of benzene and phenol....
Explanation:
The two lone pairs on the ethereal oxygen are conceived to be delocalized around the aryl ring, and as a result,

