How would i answer this???
2 Answers
Well, for starters you would draw out the acid with appropriate numbering and substitution....
Explanation:
You gots a
And we number from the carboxyl end....and we substitute the appropriate positions with ethyl and iodo functionalities....
Do you follow? If so, how many geometric isomers would this beast generate?
And now let's address the questions...
Do you agree with this treatment...
You would check Options 4 and 5.
The structure of 2-ethyl-3-iodopentanoic acid is
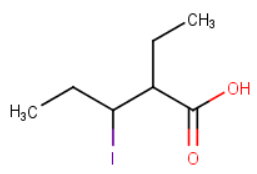
Let's examine each of the statements in turn.
1. The parent name is 3-iodopentanoic acid
FALSE The parent name is pentanoic acid.
2. It has two ethyl groups and three iodo groups
FALSE The numbers indicate the positions of the groups on the carbon chain. We use multiplying prefixes (di-, tri-, etc.) to show how many there are of each group.
3. It has only one halogen and one methyl group
FALSE It has one halogen and two methyl groups.
4. It belongs to the carboxylic acid functional group because of the ending -oic acid
TRUE If "they" give you the name first, the structure must include a carboxylic acid functional group.
5. It contains a total of seven carbon atoms
TRUE There are five carbon atoms in the main chain and two in the sidechain.


