If the volume of gas is reduced to half the the pressure will be??
1 Answer
May 18, 2018
I think it will double:
Explanation:
I would try using Boyle's Law:
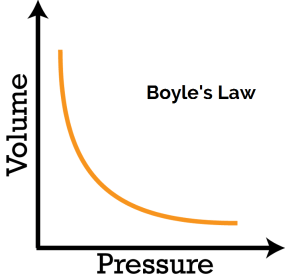
Where we have:
or, between the initial and final states:
but:
so, we get, substituting:
so:

