Indicate the molecular geometry, electronic geometry and polarity of the following molecules or ions??
Indique la geometría molecular, geometría electrónica y polaridad de las siguientes
moléculas o iones:
a) BrF5 ´
b) SO42-
Indique la geometría molecular, geometría electrónica y polaridad de las siguientes
moléculas o iones:
a) BrF5 ´
b) SO42-
1 Answer
Here's what I get.
Explanation:
a)
The Lewis structure is
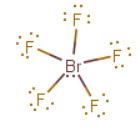
The central
The molecular geometry is square pyramidal.
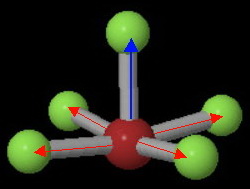
All the
The two opposing pairs in the horizontal plane cancel each other.
However, the vertical bond dipole has no opposing partner, so the molecule is polar.
**b)
The Lewis structure is
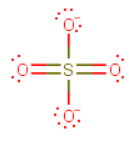
Note that this is only one of several possible Lewis structures and that all
The central
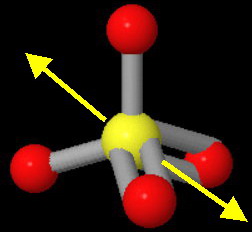
All the
The bond dipoles along the "single" bonds have a resultant in the plane of the screen and pointing toward the upper left-hand corner of the image.
The bond dipoles along the
The two resultants cancel, so the ion is nonpolar.

