K3PO4(aq)+Zn(NO3)2(aq) ?
Predict the product of the following reaction and use the proper symbol
Predict the product of the following reaction and use the proper symbol
1 Answer
Refer to the explanation.
Explanation:
Molecular equation
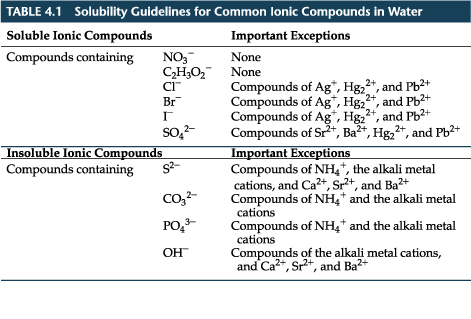
The down arrow means that the solid precipitated out of solution. This is a precipitation reaction, which is a type of double replacement reaction (also called a double displacement or metathesis) A precipitate is an insoluble solid that comes from mixing two aqueous solutions.
General form of a double replacement reaction:
where
The precipitate
The net ionic equation , which includes only the ions that reacted, is:
In order to determine which product, if any, is a precipitate, you need to refer to the solubility rules.