Mechanism of reaction of tertiary methyl chloride with #"LiAlH"_4#?
1 Answer
Apr 27, 2018
Steric hindrance prevents attack by the
Explanation:
I believe you are asking about the reaction of
It reduces alkyl halides to alkanes.
The reaction works with primary and secondary alkyl halides, but not with tertiary halides.
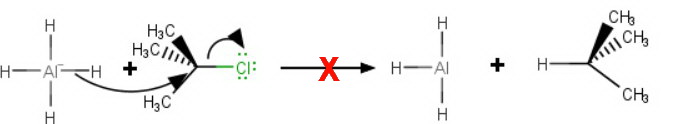
That's because the reaction is like an
Here is a space-filling model of tert-butyl chloride as an attacking
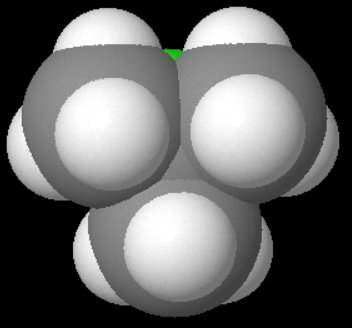
A bit of the chlorine atom is visible, but there is no room between the three methyl groups for the hydride ion to get at the carbon bearing the

