Name the following compounds???
1 Answer
Jan 15, 2018
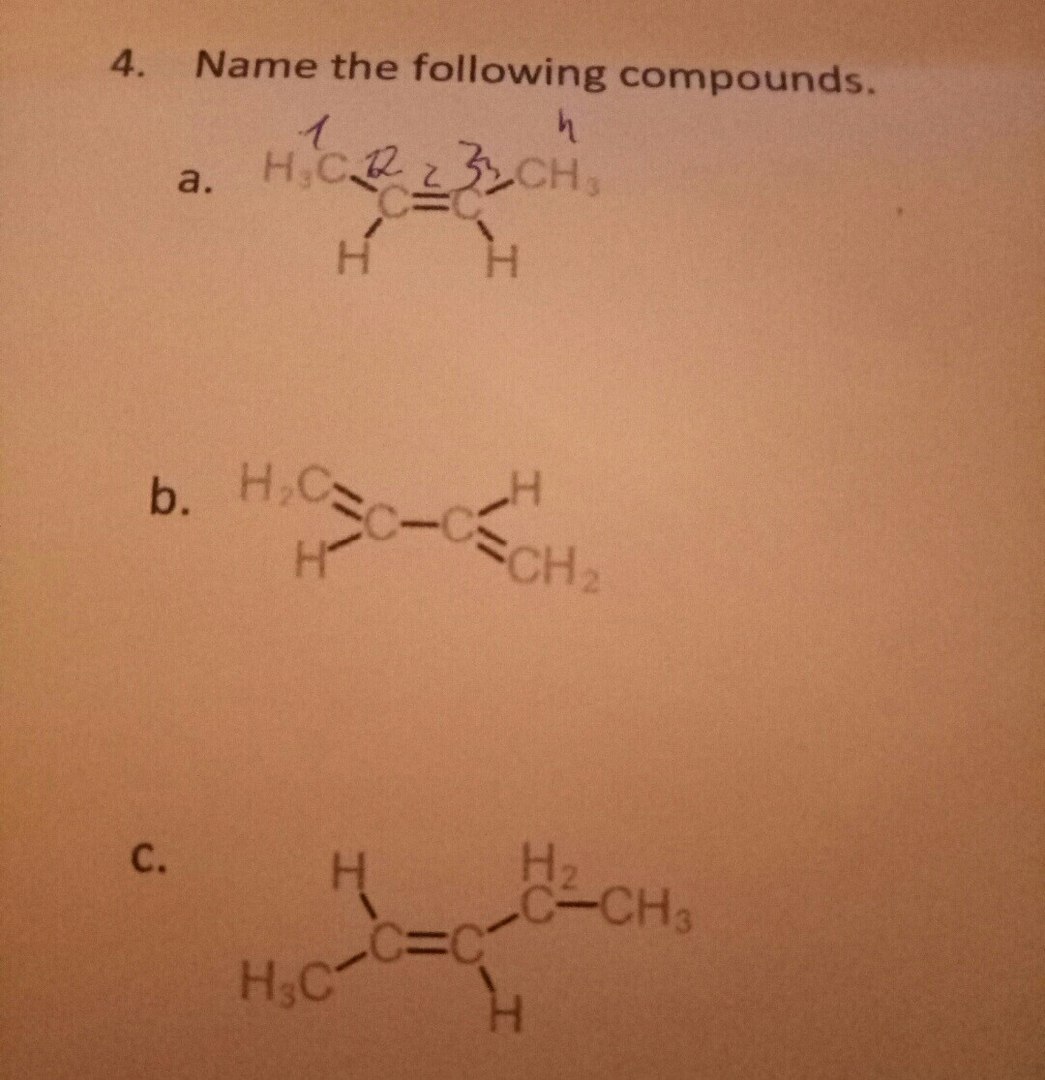
a. (
Explanation:
- Count the carbons. This is a four-carbon alkene (a butene).
- Number the carbons. Give an alkene carbon the lowest number (2).
- Name the alkene. Put the "2" immediately before the "ene" and separated from the letters by hyphens (but-2-ene).
- Assign stereochemistry. The lowest priority groups (
#"H"# ) are on the same side of the double bond. The compound is (#Z# )-but-2-ene.
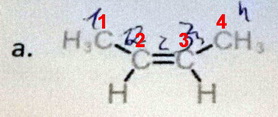
- Count the carbons. This is a four-carbon alkene with two double bonds. Insert the multiplying prefix di before the "ene" (butadiene).
- Number the carbons. Give the alkene carbons the lowest possible numbers
(1 and 3). - Name the alkene. Put the insert the numbers, separated by a comma, immediately before the "diene" and separated from the letters by hyphens. The compound is buta-1,3-diene.
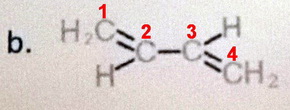
- Count the carbons. This is a five-carbon alkene (a pentene).
- Number the carbons starting from the end closest to an alkene carbon.
- Name the alkene. Put the "2" immediately before the "ene" and separated from the letters by hyphens (pent-2-ene).
- Assign stereochemistry. The lowest priority groups (
#"H"# ) are on opposite sides of the double bond. The compound is (#E# )-pent-2-ene.

