Need help with Hess law problem cant come up with third equation?
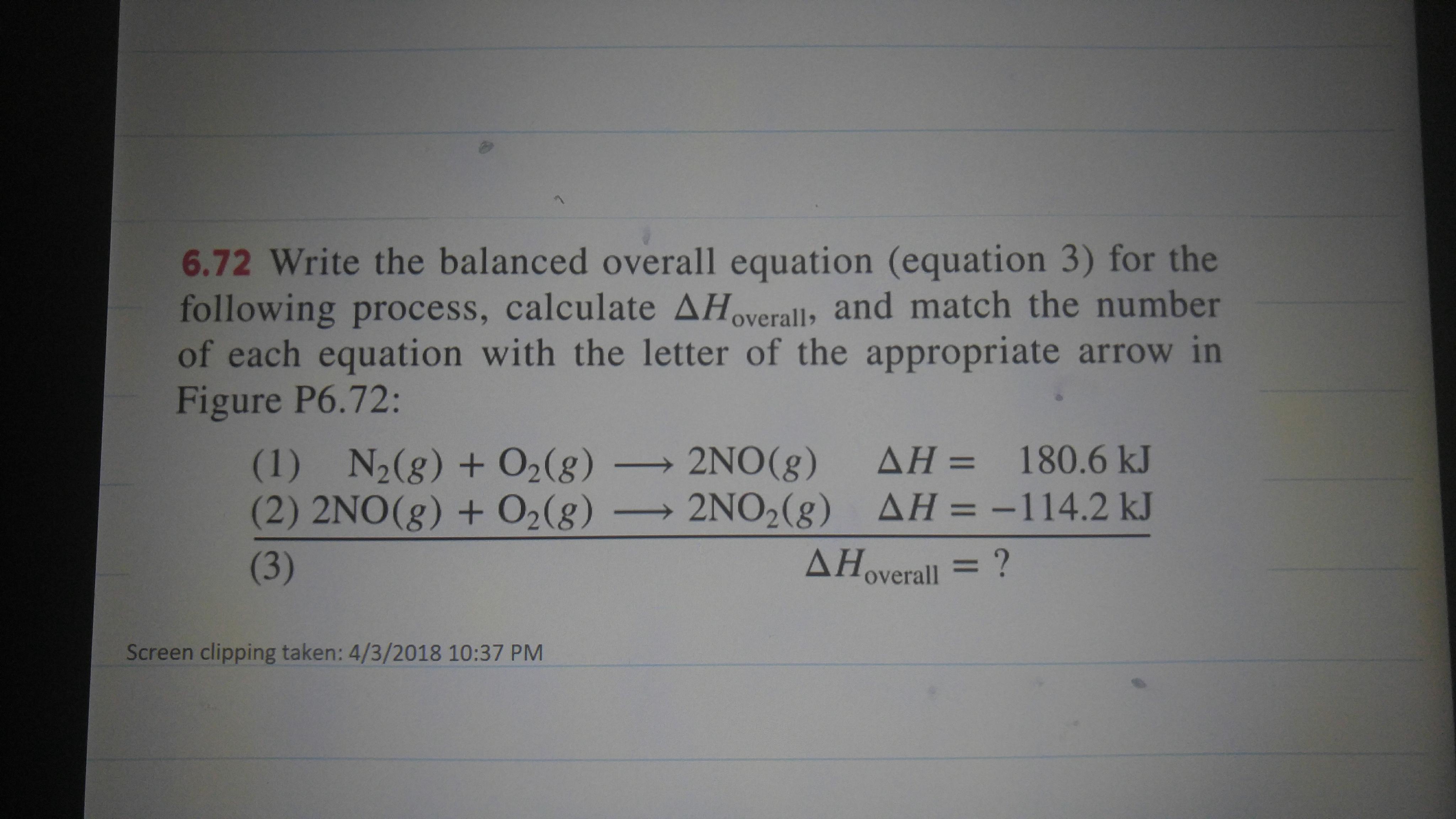
1 Answer
Apr 4, 2018
Just add the chemical equations together as you would linear equations....and in fact these are linear equations....
Explanation:
And so....
And their sum...
To give...

