Organic Chemistry Multiple Reagents Help?
Where do I even begin...
Where do I even begin...
1 Answer
Feb 27, 2018
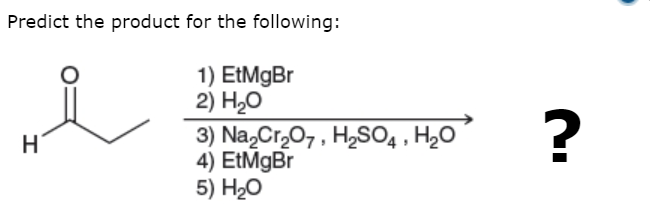
You have been given an order of operations....
Explanation:
Propionaldehyde is treated with ethyl magnesium bromide to give a secondary alkoxide...
And water work-up gives the alcohol....
Now this is oxidized up to the ketone...
And this is treated with ethyl magnesium bromide to give the tertiary alcohoxide....and aqueous work-up gives the tertiraty alcohol...
Grignard reagents are one of the few means of making

