The ordered pairs (R, t) of concentration of R (molar) in the reaction R → P with time (min) are given as (1, 0), (0.75, 0.05), (0.4, 0.12) and (0.1, 0.18). The order of the reaction is?
1 Answer
The reaction is zero-order.
When you have ordered pairs (
Thus, you use the integrated rate laws for each reaction order and see which plot gives the best result.
Zero-order reactions
The integrated rate law is
A plot of
First-order reactions
The integrated rate law is
A plot of
Second-order reactions
The integrated rate law is
A plot of
To determine the order, we plot the three different graphs and see which one gives the best straight line.
I used your data to generate the points for plotting in Excel.
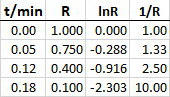
Here are the graphs.
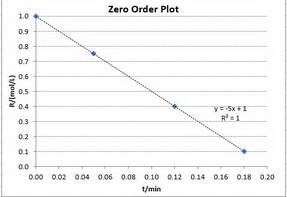
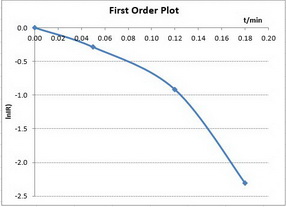
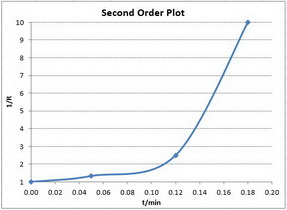
Only the first plot gives a good straight line. The best-fit straight line goes through all the points.
The reaction is zero-order.

