The rubbing alcohol used to clean test tubes is 70% isopropanol, C3H8O, by volume. 100 mL of solution contains 54.95 g of isopropanol and 29.95 g of water. The vapor pressure of water is 20 degrees celsius is .0230 atm and the vapor pressure...?
of isopropanol is .0419 atm. What is the vapor pressure of the solution at 20 degrees celsius? How would the vapor pressure change if the temperature was increased?
of isopropanol is .0419 atm. What is the vapor pressure of the solution at 20 degrees celsius? How would the vapor pressure change if the temperature was increased?
1 Answer
The vapour pressure of the solution is 0.0297 atm. The vapour pressure will increase if the temperature increases.
Explanation:
To solve this problem, we use Raoult's Law:
In symbols, the partial vapour pressure
#color(blue)(bar(ul(|color(white)(a/a)p_text(A) = chi_text(A)p_text(A)^@color(white)(a/a)|)))" "#
where
If we have two volatile components A and B, the total pressure
#p_text(tot) = chi_text(A)p_text(A)^@ + chi_text(B)p_text(B)^@#
Step 1. Calculate the moles of
Let A = isopropyl alcohol (IPA) and B = water. Then
Step 2. Calculate the mole fractions of
Step 2. Calculate the total pressure
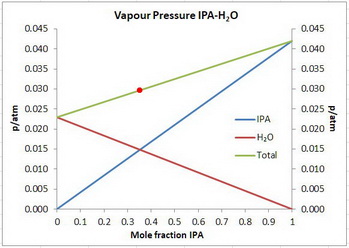
In the diagram above, you are at the position of the red dot.

