Question on the addition of HBr?
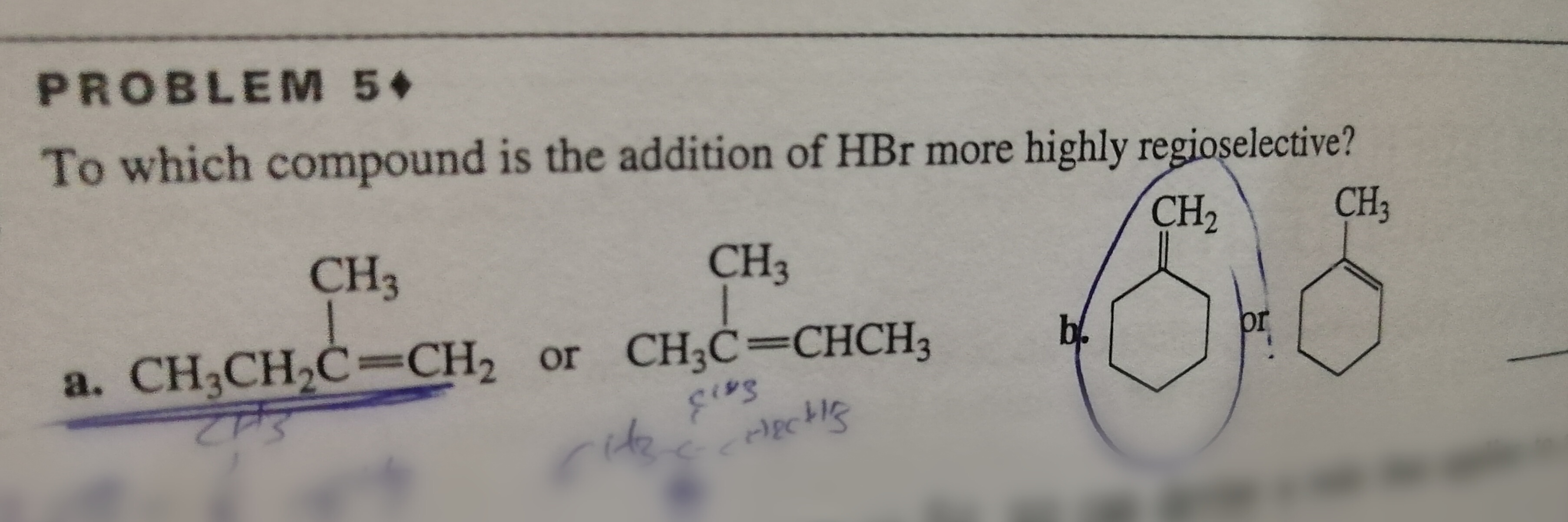
1 Answer
Apr 19, 2018
This is a weird question.
Consider

On the premise that hydrohalogenation has Markovnikov regioselectivity, compound one would have higher preference for this product,

because a carbocation intermediate on the primary carbon would be extremely unstable and would have a low yield for the other possible product.
Alternatively a secondary or tertiary carbocation don't have as large a stability difference relative to the preceding discussion.
Hence, the first has higher regioselectivity.
In

