What are the products? CH3CH(OH)CH(CH3)CH2CH3 [3-methyl-2pentanol]+ HCl=?
2 Answers
May 12, 2018
This appears to be an SN1 reaction.
Given a strong acid in water, an oxonium ion will form, leave, the molecule will stabilize by undergoing a carbocation rearrangement, and the chloride ion will attack the electrophilic carbon.

If you want the mechanism, ask!
May 13, 2018
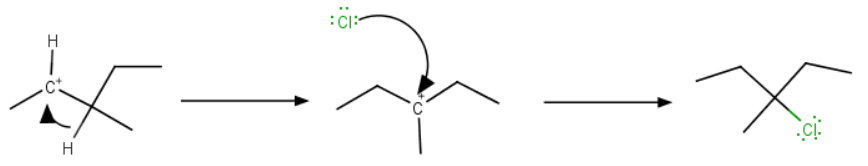
The product is 3-chloro-3-methylpentane.
Explanation:
The first steps are protonation of the
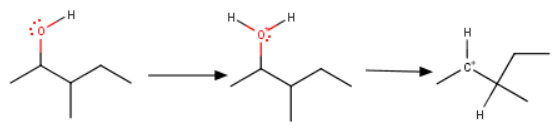
Here's the critical point to remember: Whenever a 2° carbocation can become more stable by rearranging to a 3° carbocation, it will do so.
A hydride shift forms a 3° carbocation, which then adds the