What causes the emission of radiant energy that produces characteristic spectral lines?
1 Answer
The jump of electrons between allowed orbitals accompained with the release of energy.
Explanation:
An easy example to consider (and you can find a lot of literature about it) is the Hydrogen Atom.
When a Hydrogen Atom receives energy (the right amount) its electron can jump, say, from the ground state to an Excited State.
This situation cannot remain as it is; the electron tends to go back to the ground state (that represents a condition of minimal energy for the system) and in doing so emits the surplus energy he had acquired to make the jump in the first place. This emitted energy will appear as radiation (a photon of energy equal to the energy gap between the two orbitals and given as
You can have various transitions and various spectral lines at different wavelengths.
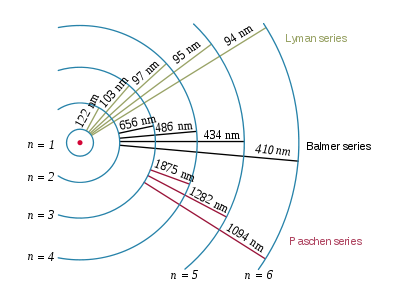
[ Transitions and emitted wavelengths for the H atom ]

