What is electron?
1 Answer
An electron is a quantum-mechanically-sized particle that is also a wave. It has:
#"rest mass"" "m_e = 9.10938356 xx 10^(-31) "kg"#
#"charge"" "q = -e = -1.60217662 xx 10^(-19) "C"#
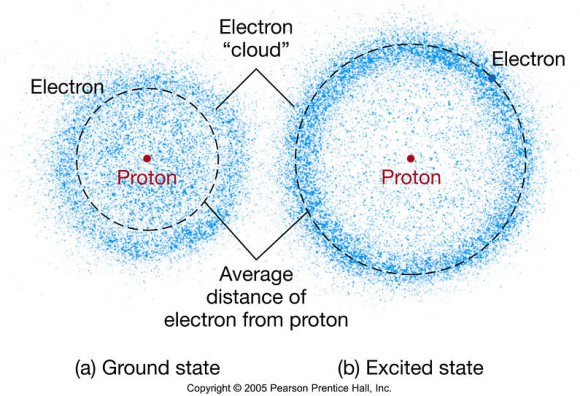
They exist outside the nucleus as an electron cloud, or region of electron density (known as an orbital), and serve as the first point of contact between two interacting atoms.
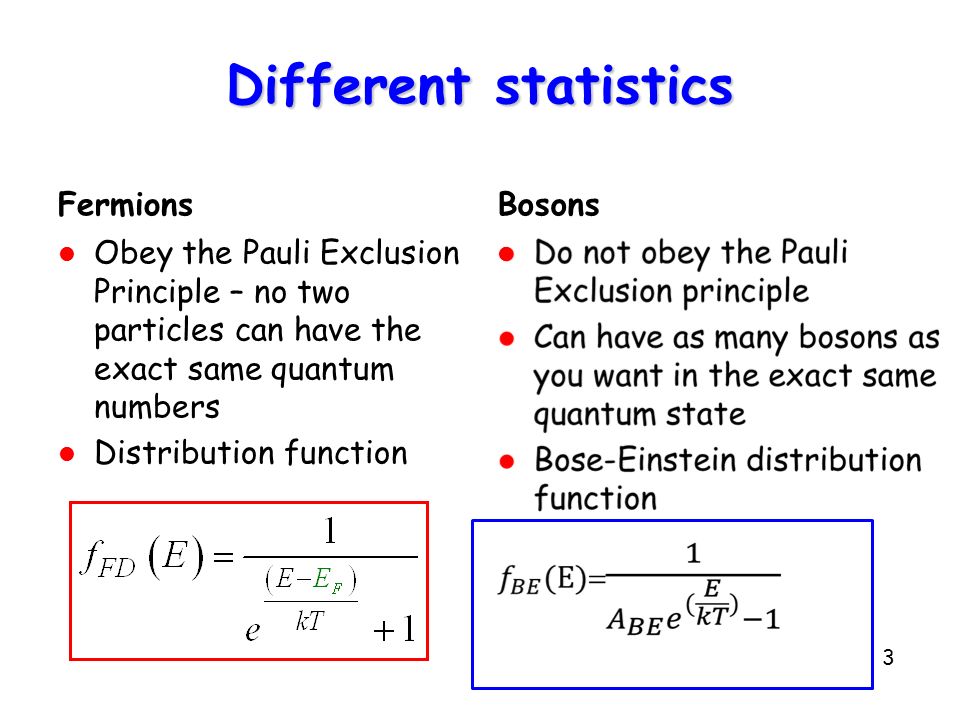
They belong to the class of particles that follow Fermi-Dirac statistics, known as fermions. That means:
- They return a negative sign for the wave function upon interchange of two electrons.
- These are spin-1/2 particles, so they have a spin quantum number of
#m_s = pm1/2# . - They follow the Pauli Exclusion Principle, so only two electrons can exist in one orbital.
They are also known as beta-minus (
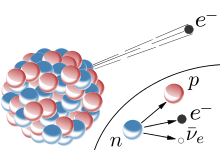
#""_0^1 n -> ""_1^1 p + ""_(-1)^(0)e + bar(nu)_e#
In nuclear chemistry, atomic units are often used:
#"rest mass"" "m_e = 5.48580 xx 10^(-4) "amu"#
#"charge" = q = -e = -"1 a.u."#